FLUORIDATED WATER:
From the Pharmacist, it has Pregnancy and Poison Warnings
From the Faucet, it has No Warnings

*Multivitamins with Fluoride. Drugs.com
A once-a-day dose of prescription fluoridated water contains 0.25 mg of fluoride, the same dose that's in 1.5 cups of fluoridated tap water. Pregnant women are advised to drink more than 5 times that amount of tap water per day.
5 times the prescribed dosage of prescription fluoridated water is an overdosage.
"If OVERDOSAGE IS SUSPECTED, seek professional assistance or
contact a Poison Control Center immediately." – FDA warning

 The American Fluoridation Society claims any warnings are only because prescription fluoridated water is far more concentrated than fluoridated tap water, but when their Information Director was challenged, he admitted that it's not the concentration of fluoride that causes adverse actions. "It is the amount of fluoride ingested."
The American Fluoridation Society claims any warnings are only because prescription fluoridated water is far more concentrated than fluoridated tap water, but when their Information Director was challenged, he admitted that it's not the concentration of fluoride that causes adverse actions. "It is the amount of fluoride ingested."
When a pregnant woman consumes fluoride, so does her baby.
Maternal fluoride supplements (1.5 mg/day) double
fetal blood concentrations of fluoride.
[European Food Safety Authority 2013]
For 25 years, it's been known that babies must
not be supplemented with fluoride in the months after birth:
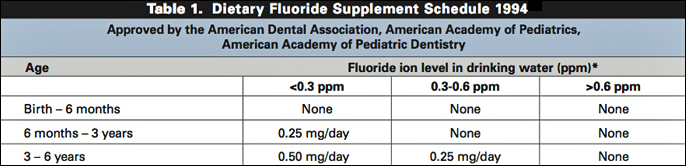
In the months before birth when their brains are most vulnerable, it's child abuse to expose babies to fluoride – especially now as laws are establishing the "personhood" of a fetus.
"The immature nervous system of a fetus is even more vulnerable to toxic exposures
than is that of an infant." – National Scientific Council on the Developing Child
EPA's Neurotoxicology Division has substantial evidence that fluoride
is a "developmental neurotoxicant," one of 22 gold standard chemicals "well
documented to alter human neurodevelopment." Others include alcohol and lead.
|
Safety Protocols Ignored When Fluoride Delivered Via Faucets
When deciding whether a child should be made to take a daily dose of 0.25 mg of fluoride, the American Dental Association (ADA) Council on Scientific Affairs said:
Healthcare providers should consider that child's "needs and preferences" before making a "judicious prescription" of dietary fluoride supplements. [2010]
ADA also stresses the importance of "developing a personalized prevention plan":
It also is critical to "assess a child's total fluoride exposure from all sources (beverages, food, toothpaste, supplements, topical applications and so forth)." [2014]
While these protocols are rarely followed, they are obviously ignored in fluoridated cities where children should swallow uncontrolled amounts of fluoride in tap water. No longer an individual, that child is now part of a vast herd. (Did somebody say, mass medication?)

|

"Unapproved prescription drugs pose significant risks to patients because they have
not been reviewed by FDA for safety, effectiveness or quality." [FDA 2008]
|
Federal Drug Administration:
Pregnancy Category for Fluoride Drugs

"Pregnancy Category C – Risk cannot be ruled out"
"Animal reproduction studies have shown an adverse effect on the fetus
and there are no adequate and well-controlled studies in humans."
– Multivitamins with Fluoride. Drugs.com
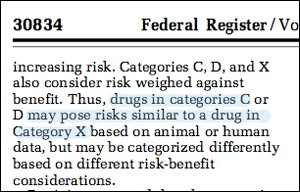 
FDA warns that a drug in Category C
"may pose risks similar to a drug in Category X,"
which carries the warning: "The risks involved in use of
the drug in pregnant women clearly outweigh potential benefits."
|
A 2017 Cochrane Systematic Review of fluoride supplementation in pregnant women revealed that "no systematic reviews have investigated the effectiveness and safety of this intervention."
|
1997: The US Food & Drug Administration
confirmed the ineffectiveness of fluoridated water.
After reviewing the best available evidence submitted by fluoridationists, the FDA would only allow this very weak health claim:
"Drinking fluoridated water may reduce the risk of
[dental caries or tooth decay]."
This is the exact same health claim the FDA allows for
eligible noncariogenic carbohydrate sweeteners like xylitol.
|
Don't Gamble with the Your Family's Health.
Avoid fluoride consumption, especially if you have dental fluorosis:
the visible evidence of your genetic susceptibility to systemic fluoride toxicity.
| 